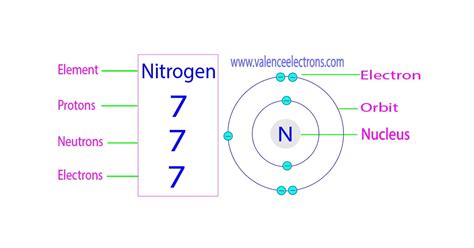
how many electrons does nitrogen have|Chemistry of Nitrogen (Z=7) : Clark Nitrogen is a colourless, odourless, unreactive gas with the atomic number 7 and the number of electrons 7. The electron configuration of nitrogen is [He] 2s2 2p3, which means it has a stable configuration of 7 electrons in . Book your Bangkok stay at 4M Pratunam Hotel with best prices only on MakeMyTrip.com. Hurry! Get , and complete your Hotel booking at the lowest price here. Check reviews, photos, contact number & address of 4M Pratunam Hotel, Bangkok here for ease of booking, and also get Free cancellation on your Hotel booking.Step 1: Create a new account on 10Cric here. Step 2: Once your account is created, navigate to the “+” button in the top right of the screen. Step 3: Deposit at least ₹1000 into your 10Cric account. At this step you will also need to fill in the Bonus Code. Write WELCOME1 where it says ‘Bonus code – enter code here’.
how many electrons does nitrogen have,Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have .
Atomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at .how many electrons does nitrogen haveAtomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at .how many electrons does nitrogen have Chemistry of Nitrogen (Z=7) Atomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at .

Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an .Therefore, the number of electrons in neutral atom of Nitrogen is 7. Each electron is influenced by the electric fields produced by the positive .

Nitrogen is a colourless, odourless, unreactive gas with the atomic number 7 and the number of electrons 7. The electron configuration of nitrogen is [He] 2s2 2p3, which means it has a stable configuration of 7 electrons in .It, therefore, has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), .Chemistry of Nitrogen (Z=7) Nitrogen is a nonmetallic element of Group 15 of the periodic table. It has the symbol N and the atomic number 7. Learn more about its history, occurrence, distribution, and commercial production .
how many electrons does nitrogen have|Chemistry of Nitrogen (Z=7)
PH0 · Protons, Neutrons, Electrons for Nitrogen (N, N3−)
PH1 · Nitrogen (N)
PH2 · Nitrogen
PH3 · Chemistry of Nitrogen (Z=7)
PH4 · 8.9.2: Chemistry of Nitrogen (Z=7)
PH5 · 2.6: Protons, Neutrons, and Electrons in Atoms